Moderna Vaccine Booster Shot Availability
The panel will consider if a booster shot of the Johnson Johnson vaccine is needed for those who received the single-dose vaccine. Older adults and 50-64 year old people with medical conditions.
Coronavirus Cdc Panel Recommends Covid Booster Shot For Vulnerable People As It Happened Financial Times
Modernas initial two-dose regime includes two 100-microgram doses.

Moderna vaccine booster shot availability. We are pleased to initiate the submission process for our booster. Weijia Jiang has the latest. MRNA vacines teach your cells how to build immunity to the virus that causes COVID-19.
Unnamed sources told Bloomberg that the FDA has seen data to suggest a half-dose would be enough to increase protection among Moderna vaccine recipients. We are pleased to initiate the submission process for our booster candidate at the 50 µg dose with the FDA. Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization.
An outside panel of the Food and Drug Administrations vaccine experts voted unanimously to endorse Modernas request to roll out booster shots of. Age 18 who live in long-term care settings. COVID-19 vaccine maker Moderna will make a third booster shot for its two-dose vaccine available to Americans by the fall CEO Stéphane Bancel said this week.
2 hours agoIf the FDA does approve a Moderna COVID-19 booster shot it will be the second of three vaccines available in the US to get the nod after the Pfizer COVID-19 vaccine booster shot which is. The Moderna CEO says booster shots will be available by late summer or early fall. COVID-19 Vaccine booster shots are available for the following Pfizer-BioNTech vaccine recipients who completed their initial series at least 6 months ago and are.
Many Americans who received a Moderna COVID-19 vaccine could soon have access to a booster shot after an FDA advisory panel unanimously endorsed a third dose. Booster shots are now available to select individuals who received the Pfizer-BioNTech vaccine six months after their second dose. Age 18 who have underlying medical conditions.
Food and Drug Administration was leaning toward authorizing half-dose booster shots for those who received Modernas two-shot mRNA vaccine. Plans to authorize a Moderna COVID-19 booster vaccine are ramping up and now the Food and Drug Administration is reportedly leaning toward a half dose as a booster. EUAs for COVID-19 vaccines manufactured by Moderna and Johnson Johnsons Janssen remain in effect.
Booster Shots Coronavirus Coronavirus Vaccine Dr. Bloomberg citing people familiar with the matter reported last week that the US. The Pfizer vaccine is the first to receive FDA approval while the Moderna and Johnson Johnson vaccines are available under an emergency use authorization.
As for the dose initial Moderna vaccination consists of two 100-microgram shots. Learn more about the facts behind the COVID-19 Vaccine. Pfizer for comparison has two 30-microgram doses to start vaccination.
But Moderna says a single 50-microgram shot should be enough for a booster. The company said that would trigger fewer uncomfortable shot reactions such as fever and achiness while also leaving more vaccine available for the global supply. FDA reviewers noted that a half-dose booster shot of the Moderna vaccine topped off the level of antibodies the most easily measurable barometer of immunity.
Booster Shots Are Only Available for Some Pfizer-BioNTech Vaccine Recipients Only certain populations initially vaccinated with the Pfizer-BioNTech vaccine can get a booster shot at this time. Vaccinesgov helps you find clinics pharmacies and other locations that offer COVID-19 vaccines in the United States. So the booster shot would be a 50-microgram dose.
The group includes people with a weakened immune system and patients with a history of cancer and organ transplant. 18 the Department of Health and Human Services HHS issued a press release stating that preparations were underway for booster shots of messenger RNA mRNA vaccines to be made available. Panel Recommends Booster for Many Moderna Vaccine Recipients.
Moderna submitted data to the FDA seeking evaluation for its booster shot on Sept. Age 18 who live in high-risk settings. 65 years and older.
2 days agoVaccine experts advising the Food and Drug Administration voted 19-0 to recommend authorization of an extra dose of Modernas Covid-19 shot a key step in making booster doses available. Up to 6 cash back Emergency use authorization EUA continues for ages 12 to 15. The booster shot is a 30.
Made a third shot available. Moderna submitted data to the FDA seeking evaluation for its booster shot on Sept. Mallika Marshall Moderna BOSTON CBS An FDA panel voted yes on Thursday to recommend a third dose of the Moderna vaccine.
There is currently no information on the availability of the JJs booster dose or additional dose. So far Americans who received the Moderna COVID-19 vaccine cant get booster shots but could that soon be changing. For children ages 12 to 15.
FDA panel recommends Moderna booster shots 0320. US regulators are yet to decide on the booster doses for the Moderna and JJ Booster doses. Ad mRNA vaccines were held to the same safety effectiveness standards as all other vaccines.
Age 18 who work in high-risk settings. Those eligible for the extra shot would include adults over 65 and others at high risk the same groups now eligible for a.
/cdn.vox-cdn.com/uploads/chorus_asset/file/22293502/Mass_Vax_Site_Presser_sg_09.jpg)
Moderna Booster Shot May Only Be Available In Half Doses Here S Why Deseret News
Covid Booster Shots Everything You Need To Know The Brink Boston University

Moderna Covid Vaccine Booster Produces Robust Response Against Delta

Moderna Covid Vaccine Booster Shot Update New Fda Panel Details To Know Cnet
Pfizer S Covid Booster Shots Who S Eligible Vaccine Availability And More Cnet

Covid Booster Shots Everything You Need To Know The Brink Boston University

Pfizer Biontech Booster Shots Approved Ohsu News

Covid 19 Vaccine Update Lasalle County
Q A What To Know About Waning Vaccine Effectiveness And Booster Shots Uva Today

Boosters For Moderna And J J Recipients Not Up For Debate At C D C Panel The New York Times

Moderna Submits Preliminary Data To Fda For Covid 19 Booster Shot
Booster Shot U S Begins Study Testing Mix And Match Covid Vaccine Doses

Moderna Plans To Have Covid Vaccine Booster Shot Ready By Fall Cbs News

Moderna Seeks Fda Approval For Booster Shots Of Its Covid 19 Vaccine Healthcare Finance News
Booster Shots Third Doses What You Should Know By Washington State Department Of Health Public Health Connection Sep 2021 Medium
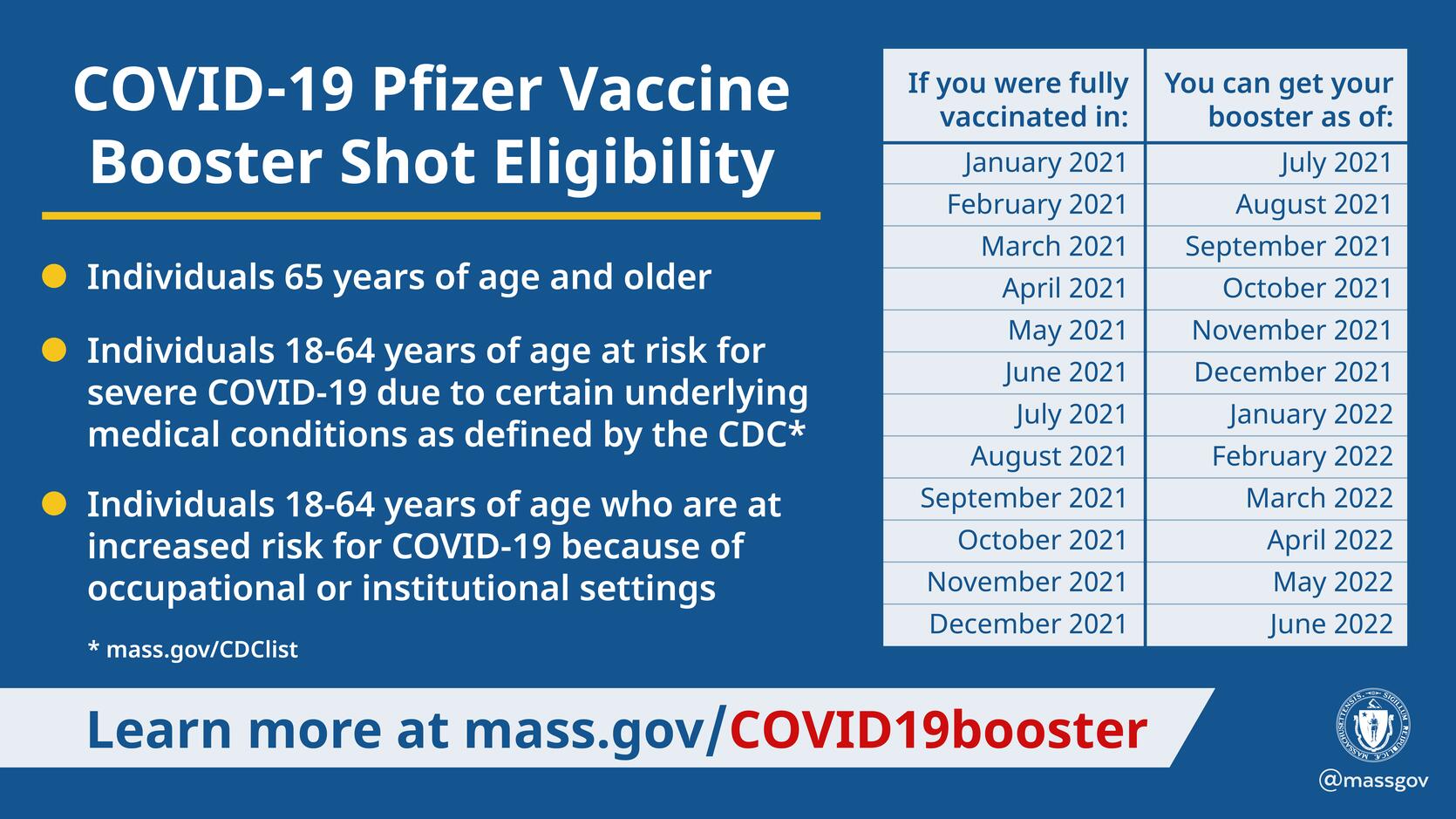
Baker Polito Administration Provides Update On Pfizer Covid 19 Booster Availability Mass Gov

Moderna Asks Fda To Authorize A Booster Shot Of Its Covid 19 Vaccine Coronavirus Updates Npr

What S The Difference Between A Booster And A 3rd Dose Of The Covid Vaccine Wgn Tv